Pediatric-inspired asparaginase-containing regimens are recommended treatment options for frontline treatment of AYA patients with ALL1
ALL outcomes have improved over the past 40 years in pediatric patients, while AYA patients* have inferior outcomes compared to the pediatric population2,3
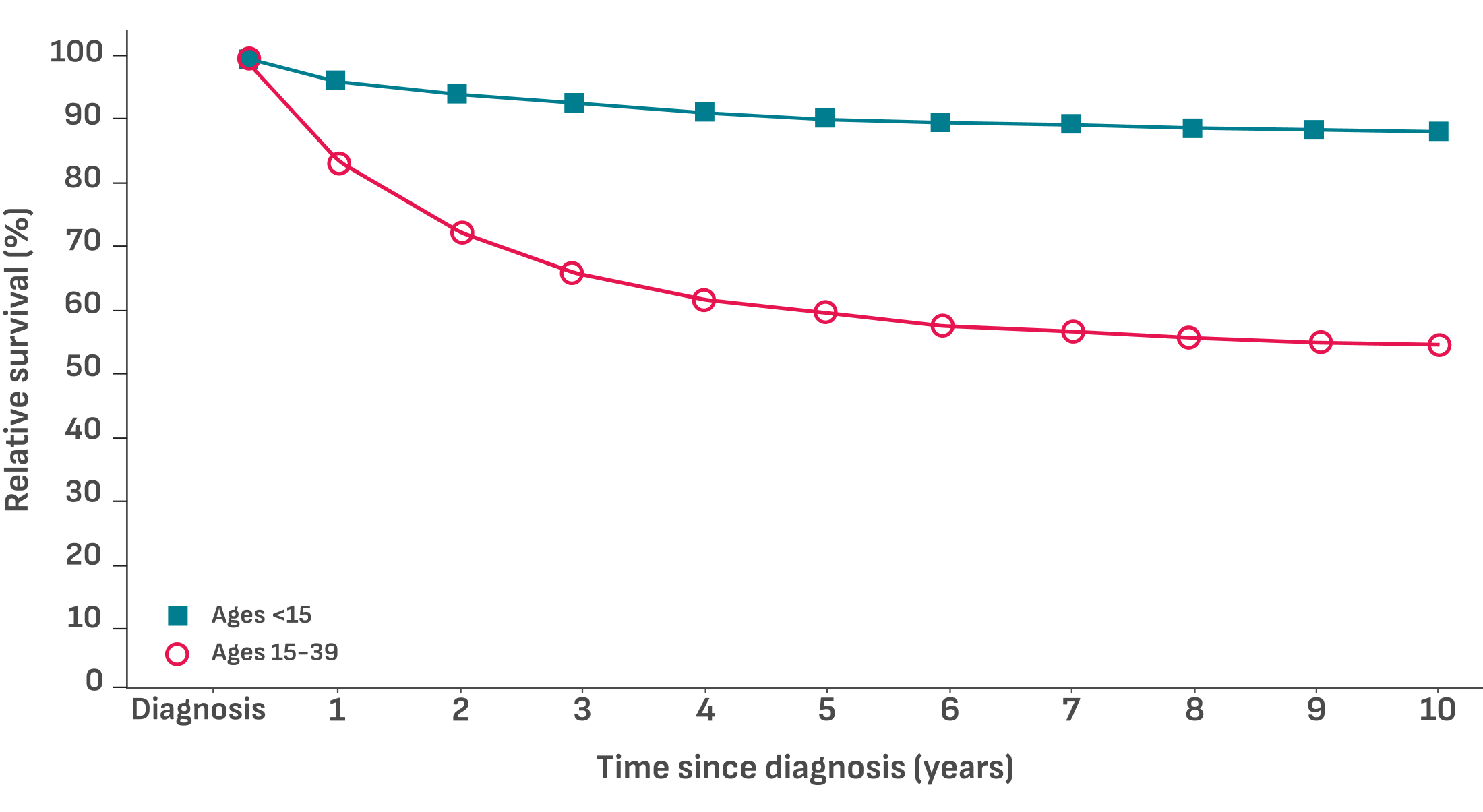
Acute lymphoblastic leukemia relative survival rates by time since diagnosis in pediatric and AYA patients4
*AYA patients are defined as patients aged 15 to 39 years at diagnosis.1
- KM curve of ALL relative survival rates by time since diagnosis, including all stages by age, both sexes, and all races. Data from the SEER registries, 2000-20204
AYA survival rates decline with increasing age4
- 92.3% (95% CI: 91.6-93.0) 5-year relative survival rate in patients aged <15 years
- 66.9% (95% CI: 65.1-68.6) 5-year relative survival rate in patients aged 15 to 39 years
Treating AYA patients can be challenging due to several factors5-7
- Differences in disease biology5,6
- Lack of uniform treatment strategy5
- Underrepresentation in clinical trials5
- Treatment compliance/adherence7
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for ALL recommend as preferred regimens certain pediatric-inspired asparaginase-containing regimens in the treatment of AYA patients with:
- Ph- B-ALL (frontline therapy)1
- T-ALL (frontline therapy)1
- Pediatric-inspired regimens are not recommended for Ph+
NCCN® makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.1
The majority of frontline therapy protocols recommended by the NCCN Guidelines® for AYA patients with Ph-negative ALL include asparaginase as part of the treatment regimen.1
Preferred multiagent frontline therapy regimens for AYA patients (ages 15-39) are based on protocols and data from multi-institutional studies1,a
aThis age range (15-39) is not a firm reference point because some of the recommended regimens have not been comprehensively tested across all ages.
bPediatric-inspired regimen.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 22, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.1
Pediatric-inspired regimens can improve AYA outcomes8
Review the most recent data supporting the use of asparaginase in AYA patients
Protocol
Ph- B-ALL
T-ALL
CALGB 10403b
COG AALL 0434b
DFCI ALLbbased on DFCI Protocol 00-01
GRAALL-2005b
PETHEMA ALL-96b(if aged <30 years)
Dose-adjusted Hyper-CVAD
USC/MSKCC ALLbregimen based on CCG-1882 regimen (if aged ≥18 years)
Linker 4-drug regimen(if aged <60 years)
ECOG 1910
Ph- B-ALL
CALGB 10403b
COG AALL 0434b
DFCI ALLbbased on DFCI Protocol 00-01
GRAALL-2005b
PETHEMA ALL-96b(if aged <30 years)
Dose-adjusted Hyper-CVAD
USC/MSKCC ALLbregimen based on CCG-1882 regimen (if aged ≥18 years)
Linker 4-drug regimen(if aged <60 years)
ECOG 1910
T-ALL
CALGB 10403b
COG AALL 0434b
DFCI ALLbbased on DFCI Protocol 00-01
GRAALL-2005b
PETHEMA ALL-96b(if aged <30 years)
Dose-adjusted Hyper-CVAD
USC/MSKCC ALLbregimen based on CCG-1882 regimen (if aged ≥18 years)
Linker 4-drug regimen(if aged <60 years)
ECOG 1910
